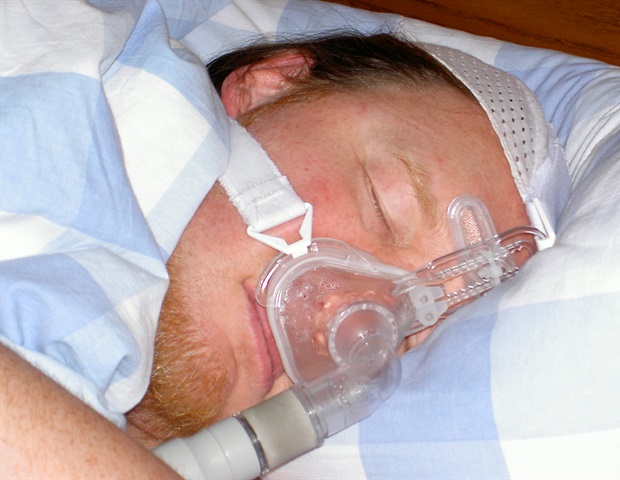
Researchers at University of California San Diego School of Medicine and international collaborators have led a worldwide, advanced study demonstrating the potential of tirzepatide, known to manage type 2 diabetes, as the first effective drug therapy for obstructive sleep apnea (OSA), a sleep-related disorder characterized by repeated episodes of irregular breathing due to complete or partial blockage of the upper airway.
The results, published in the June 21, 2024 online edition of New England Journal of Medicine, highlight the treatment’s potential to improve the quality of life for millions around the world affected by OSA.
This study marks a significant milestone in the treatment of OSA, offering a promising new therapeutic option that addresses both respiratory and metabolic complications.”
Atul Malhotra, MD, lead author of the study, professor of medicine at University of California San Diego School of Medicine and director of sleep medicine at UC San Diego Health
OSA can result in reduced oxygen levels in the blood and can also be associated with an increased risk of cardiovascular complications, such as hypertension and heart disease. Recent studies, also led by Malhotra, suggest that the number of OSA patients worldwide is close to 936 million.
Conducted in two Phase III, double-blinded, randomized, controlled trials, the new study cohort involved 469 participants diagnosed with clinical obesity and living with moderate-to-severe OSA. They were recruited from sites in nine different countries, including the U.S., Australia and Germany. Participants either used or did not use continuous positive airway pressure (CPAP) therapy, the most common sleep apnea treatment which uses a machine to maintain an open airway during sleep, preventing interruptions in breathing. Patients were administered either 10 or 15 mg of the drug by injection or a placebo. The impact of tirzepatide was evaluated over 52 weeks.
Researchers found that tirzepatide led to a significant decrease in the number of breathing interruptions during sleep, a key indicator used to measure the severity of OSA. This improvement was much greater than what was seen in participants that were given a placebo. Importantly, some participants that took the drug reached a point where CPAP therapy might not be necessary. Considerable data suggest that a drug therapy that targets both sleep apnea and obesity is beneficial rather than treating either condition alone.
Additionally, the drug therapy improved other aspects related to OSA, such as reducing the risk factors of cardiovascular diseases and improved body weight. The most common side effect reported was mild stomach issues.
“Historically, treating OSA meant using devices during sleep, like a CPAP machine, to alleviate breathing difficulties and symptoms,” Malhotra said. “However, its effectiveness relies on consistent use. This new drug treatment offers a more accessible alternative for individuals who cannot tolerate or adhere to existing therapies. We believe that the combination of CPAP therapy with weight loss will be optimal for improving cardiometabolic risk and symptoms. Tirzepatide can also target specific underlying mechanisms of sleep apnea, potentially leading to more personalized and effective treatment.”
Malhotra adds that having a drug therapy for OSA represents a significant advancement in the field.
“It means we can offer an innovative solution, signifying hope and a new standard of care to provide relief to countless individuals and their families who have struggled with the limitations of existing treatments,” said Malhotra. “This breakthrough opens the door to a new era of OSA management for people diagnosed with obesity, potentially transforming how we approach and treat this pervasive condition on a global scale.”
Next steps include conducting clinical trials to examine longer term effects of tirzepatide.
Co-authors of the study include: Ronald Grunstein, University of Sydney; Ingo Fietze, University Hospital Berlin; Terri Weaver, University of Illinois Chicago; Susan Redline, Ali Azarbarzin, and Scott Sands, Harvard Medical School; Richard Schwab, University of Pennsylvania; and Julia Dunn, Sujatro Chakladar, Mathijs Bunck, and Josef Bednarik, Eli Lilly and Company.
Funding support for the study came, in part, from Eli Lilly and Company.
Source:
Journal reference:
Malhotra, A., et al. (2024) Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. New England Journal of Medicine. doi.org/10.1056/NEJMoa2404881.