Fitness
From toxic fungus to soy sauce superstar

Nearly 9,000 years ago, around the time that humans were first domesticating corn and pigs, some people in China were taming fungi.
One such fungus, the mold Aspergillus oryzae, would go on to become a culinary superstar. Through fermentation of raw ingredients like soybeans or rice, A. oryzae helps to bring us soy sauce, sake and several other traditional Asian foods. It does so by breaking down proteins and starches so that other microbes can finish off the fermentations.
But A. oryzae wasn’t always so obliging. The wild version of the mold makes potent toxins that can poison the consumer and lead to cancer in the liver and other organs. Plus, it’s a destructive agricultural pest that causes millions of dollars in damage each year to crops like peanuts and corn.
JOHN GIBBONS / VANDERBILT UNIVERSITY
The food fermenter Aspergillus oryzae (left) is thought to be a domesticated version of Aspergillus flavus (right), which makes aflatoxins that can contaminate crops, poison people and cause cancer. The two colonies shown here are visually quite different, but the forms of these two molds vary widely, making it hard to tell them apart.
What changed? Research is steadily revealing how the fungus transformed from a dangerous, toxic mold into a superior tool of food biotechnology that thrives in human-made environments. And as scientists study A. oryzae, they’re learning more about the process of domestication in microbes in general — which still remains in many ways mysterious.
“Almost everything we know comes from plants and animals,” microbial genomicist John Gibbons of UMass Amherst says of domestication. “You can see the difference between dogs and wolves, between corn and teosinte, but you can’t really see the differences between microbes … because most of it is changes in metabolism.”
A master digester
A. oryzae belongs to a family of fungi in a larger group known as the blue and green molds. Some 40 percent of the family’s species are in the genus Aspergillus, so named because the slender stalks and poofy tips of its spore-producing structures resemble an aspergillum, the holy water sprinkler used in some Christian denominations. The genus has several high-profile members, including helpful industrial species that crank out useful chemicals such as medicines or ferment foods, as A. oryzae does.
Known as the koji mold, A. oryzae is a master digester. In the first stage of soy sauce production, A. oryzae tackles the starter ingredients, typically soybeans and wheat; in sake production, it goes to work on rice. The mold’s digestive enzymes — proteases and amylases — break down the proteins and starches into simpler molecules that will be fermented by yeasts later on. The mold “smells like this wonderful mix of mushroom and grapefruit, and a little bit sour as well,” says microbiologist Benjamin Wolfe of Tufts University near Boston.
Other Aspergillus species are menaces — among them, Aspergillus flavus, the Mr. Hyde to A. oryzae’s Dr. Jekyll. A. flavus makes potent poisons called aflatoxins that, when ingested, are metabolized by the liver into compounds that damage DNA and otherwise mess with cellular functioning. It infects a variety of crops — corn, wheat, cassava, chili pepper, peanut, rice, sesame, sunflower seed and more. It can contaminate plants both before harvest and after, when crops are stored or shipped. The toxins can even contaminate the milk of animals that eat tainted feed. Despite various control measures, sporadic aflatoxin outbreaks poison and kill people and pets around the globe.
Scientists have long recognized that the hazardous A. flavus and the food fermenter A. oryzae are very close relatives — the two can appear identical in color and texture, or look very different from each other, making it tricky to tell them apart. Early investigations of their DNA reported remarkable similarity, and a 1998 study of a handful of genes from each fungus concluded that A.oryzae evolved via domestication from A. flavus.
But A. oryzae doesn’t make aflatoxin and has been safely used as a food fermenter for thousands of years. Now scientists have begun to pinpoint the specific tweaks that that led to the major overhaul of the mold’s metabolism.
A pivotal genetic deletion
Scientists had long been keen to establish genetic proof that A. oryzae couldn’t make aflatoxin, partly for reassurance that the mold is, and would remain, safe for fermenting food. Over the years, they have documented numerous large- and small-scale destructive changes in the cluster of more than two dozen genes that the fungus’s ancestor employed to make the toxin.
In one recent study, for example, scientists compared the genome of A. oryzae 14160, an industrial strain from China, with the genome of A. oryzae RIB40, a strain that was sequenced in 2005. In a report published in Frontiers in Microbiology in 2021, the team found that more than half of the aflatoxin gene cluster was deleted in strain 14160, while strain RIB40 has mutations in key genes here and there.
But from strain to strain to strain, there’s one deletion in the aflatoxin gene cluster that consistently appears, says Gibbons, who led the 2021 analysis with then-graduate student Katherine Chacón-Vargas (the group has been analyzing hundreds of strains of the molds). This finding suggests that at some point, a strain of wild A. flavus mold acquired the deletion, which rendered it harmless. After that, other genetic changes — mutations, deletions, other alterations — freely accumulated in the aflatoxin genes since they were no longer being used.
Domestication would have ensured that the harmless trait remained, says Gibbons. That’s because aflatoxin is a defensive compound the mold uses to kill other microbes. Since other microbes — specifically, yeasts — are part of the fermentation process for making soy sauce or the rice wine sake, the only fermentations that would work would be those in which Aspergillus toxins weren’t present to kill off the yeasts.
And in the cushy domesticated environment, the toxins aren’t important anyway. “You have this really stable food source all the time and there’s no longer a reason to produce defense chemicals because there’s enough food for everybody,” Gibbons says.
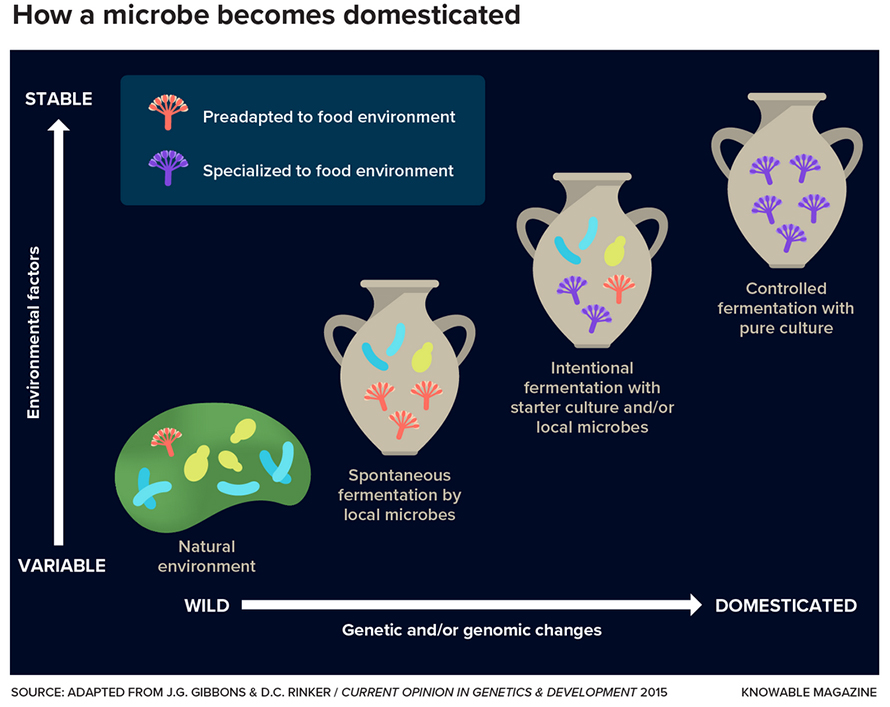
Scientists think that the domestication of a microbe could go something like this: It exists, along with other microbes, in the wild, where environmental factors such as temperature and humidity vary (bottom left). Over time, it becomes adapted to the stable, comfortable food environment (middle) and eventually exists as a pure culture in a very controlled environment.
The loss of the ability to make aflatoxin probably paved the way for the fungus to ramp up its starch-digesting abilities, Gibbons adds. That’s because defense chemicals are expensive to make. “If they lose the ability to produce those toxins, it actually saves them a lot of energy that they can put into primary metabolism, like digesting starches and sugars and proteins,” he says.
Research suggests that this dialed-up ability to digest starch evolved over and over again. Back in 1989, for example, long before genome sequences were available for any Aspergillus species, several groups of scientists used methods to show that A. oryzae had multiple copies of the gene coding for alpha-amylase, the starch-digesting enzyme; two strains of the fungus had two copies while two other strains had three.
Researchers have since looked more closely and at more strains and found all sorts of variations on this theme. Strain RIB40, for example, has alpha-amylase genes on chromosomes 2, 3 and 5, while Gibbon’s team recently reported that the industrial strain from China, 14160, has two copies on chromosome 2 and a third copy on chromosome 6.
These kinds of changes also probably happened many times in the wild, says Gibbons, though before domestication, they weren’t retained because they weren’t of use. “But in the food environment, the more of these alpha-amylase genes you have, the more of this enzyme you’re producing,” he says. We humans would then have selected the starch-digesting powerhouse microbes in our domestication for fermentations.

NATIONAL DIET LIBRARY, JAPAN
This illustration, from Koeki Kokusanko, an agricultural book of Japan’s Edo era (1603-1867), shows the production of soy sauce. At top right, the koji mold is being mixed with soybeans and wheat on a large mat in the first of two ferments required to make the condiment.
Domestication of A. oryzae could have happened very quickly if research on Penicillium species, another famous mold in the Aspergillus family, is anything to go by.
P. camemberti, which is responsible for the white rind and distinctive smell of Camembert and Brie cheeses, is thought to have evolved from P. commune, a darkly pigmented, toxin-producing species with a musty odor. When Wolfe’s group at Tufts took a wild P. commune strain and another non-cheese Penicillium strain and serially grew them on cheese, after only eight generations — a period of a few weeks — the wild strains showed signs of domestication. Reporting in the journal mBio in 2019, the team found that the molds’ ability to make pigment and toxins diminished. At the same time, they lost their musty odor, acquiring the buttery, cheesy aromas characteristic of their domesticated relatives.
The human factor in fermentation
When contemplating the steps in the taming of A. oryzae, it’s useful to remember that fermentation and human evolution have probably always been intertwined, says microbial geneticist Kevin Verstrepen of VIB and Leuven University in Belgium.
For example, it’s easy to imagine early hominids eating fruit that had been visited by yeast and fermented into an alcoholic mash, and for humans to have recognized the merits of such fruit, both for its mind-altering effects and disinfectant qualities. “I wouldn’t be surprised if those things were discovered quite quickly,” says Verstrepen.
In the case of Aspergillus, spores are constantly drifting about — we inhale upwards of 200 per day, researchers estimate — and they will grow if they settle in a warm, humid spot. A recent reconstruction of the Aspergillus family tree by evolutionary biologist Antonis Rokas of Vanderbilt University suggests that A. flavus and some version of its domesticated counterpart, A. oryzae, last shared an ancestor roughly 3.8 million years ago. A. oryzae is naturally fond of rice, and versions of A. flavus that didn’t make aflatoxin were likely present on wild rice plants consumed by early humans.
With the advent of farming in the Neolithic some 12,000 or so years ago, domestication became a full court press. As people settled in communities and began regularly planting crops and keeping animals, there would have been an excess, perhaps for the first time, of grain or milk or meat. Fermentation provided a way to keep food past harvest and prolong shelf life.
“One of the best examples is raw milk — it goes bad in a day or so at room temperature,” says Gibbons. “But if you ferment it into a hard cheese, you can travel around with it in your pocket at room temperature for a month.”
An early example of people intentionally fermenting foods — very likely using Aspergillus — comes from the Neolithic village Jiahu in Henan province in China, a site with artifacts suggesting domesticated rice and early musical instruments. In 2004, a team reported that pottery shards from the site contained residues of a fermented drink of rice, honey and fruit — basically, a rice wine or “proto-sake,” says Gibbons. Scientists have since investigated residues in vessels from two other early Neolithic sites in China and found traces of fungi, including some that are startlingly like our hero, the koji mold.
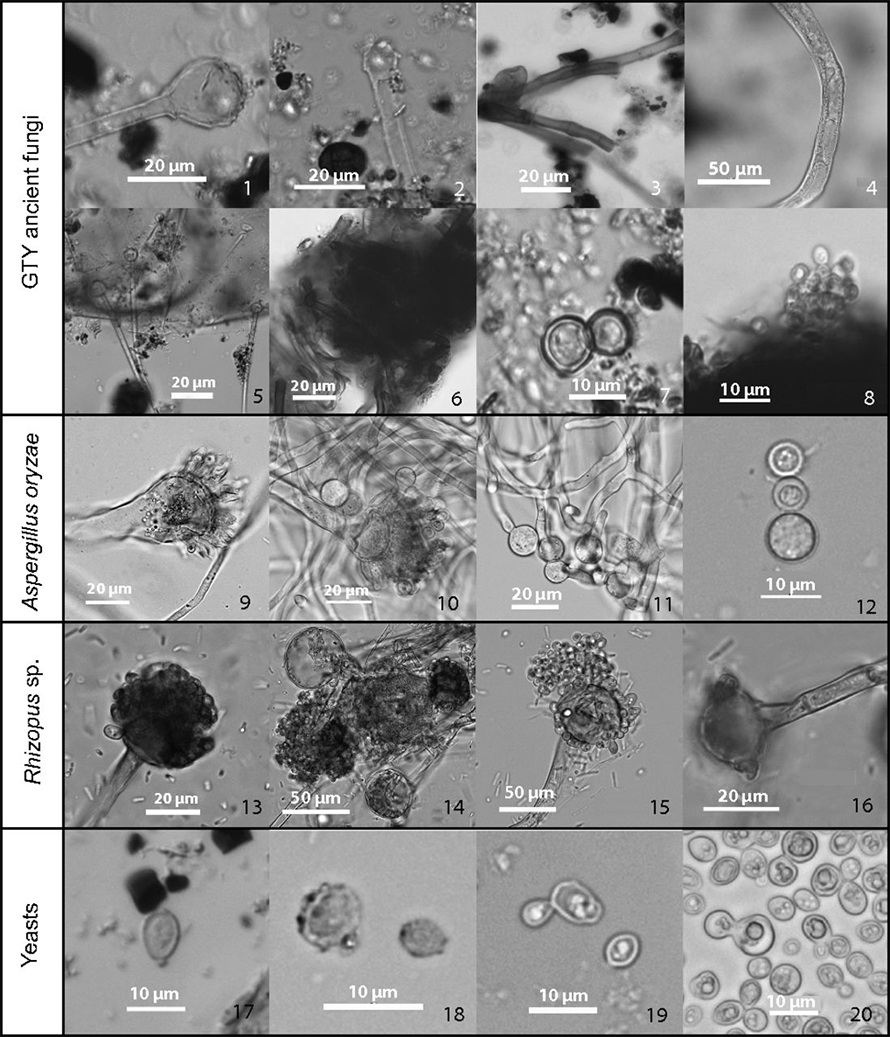
L. LIU ET AL / PNAS 2019
Analyses of residue on pottery shards from the neolithic site of Guantaoyuan in China revealed bits of microbial structures (top two rows, GTY ancient fungi). These ancient microbes have a striking resemblance to the fungi Aspergillus oryzae (third row), Rhizopus (fourth row) and yeasts (bottom row), which are used to make various fermented foods and drinks.
Initially, people probably relied on spontaneous colonization by A. oryzae and other microbes but at some point, “back-slopping” developed, wherein a portion of a previous ferment is used to start a new one, like a sourdough starter is used for bread. This intentional fermentation with A. oryzae appears to have been happening as early as 2,300 years ago: The mold gets a mention in the ancient Chinese text Zhouli (Rites of the Zhou dynasty) that dates to 300 BCE. Some time later, people began breeding A. oryzae on steamed rice; its spores were then separated from the grain with a silk sieve and dried for use as needed.
Verstrepen is fond of telling his students that beer yeasts, living year-round in their vats where they are warm and well-fed, are like dogs, while wine yeasts, which are harnessed during the harvest but may intermingle with wild species in the intervening months, are like cats.
Today, says Rokas, A. oryzae is like a dog. There are numerous bred strains that people can order depending on their specific fermenting needs. But for a long time, there would have been unfettered variety floating around — lots of A. oryzae/A.flavus strains with defunct toxin genes and differing abilities to digest starch, and a matter of fortune which ended up in your soy sauce or sake brew. The mold of the ancients, Rokas says, “must have been more catlike.”
This article is reprinted from Knowable Magazine. Read the original here.










