Hearing loss is one of the most prevalent disorders and it is estimated that over one billion people suffer from hearing loss and approximately one-two children in every 1,000 births are born with congenital hearing loss. It is not therefore surprising that screening for newborns is an essential component of newborn screening programmes.
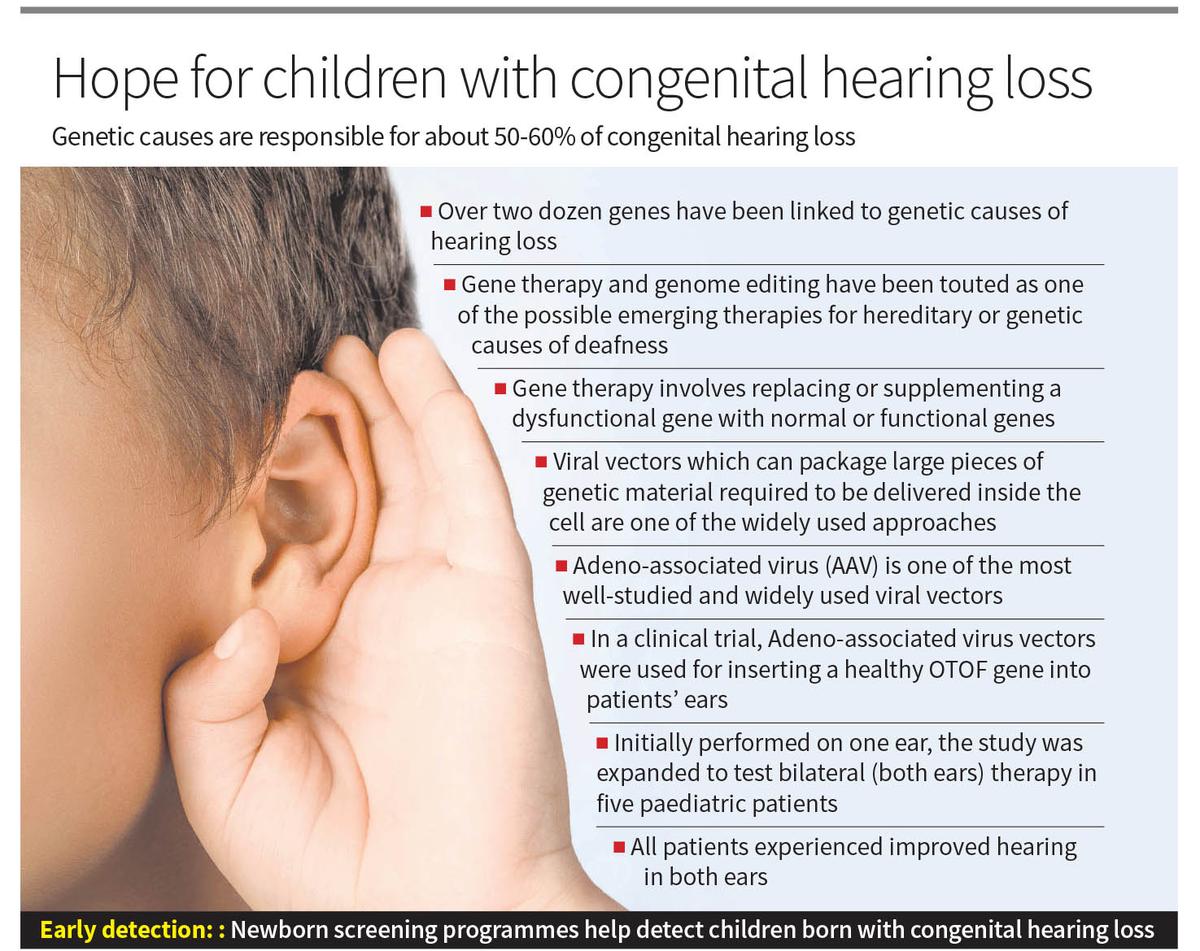
Hearing loss is a complex condition that can result from a variety of environmental and genetic factors including ear infections. Often, hearing loss serves as symptoms indicating defects or pathologies in the ear’s process that converts sound into electrical signals sent to the brain. It is widely estimated that a significant majority, amounting to approximately 50-60% of congenital hearing loss cases, are attributed to genetic causes. Among the various populations, genetic variants play a significant role. For example, mutations in the GJB2 gene are the most common genetic cause of hearing loss in Caucasian, Asian and Hispanic populations. In Africa, the MYO15A and ATP6V1B1 genes are more frequently implicated. In total, over two dozen genes have been linked to genetic causes of hearing loss. Besides genomic mutations, mitochondrial genetic defects can also lead to hearing impairment. Genetic variants could also play a role in the complex interplay with other factors, like medications. For instance, a prevalent genetic defect in the mitochondrial MTRNR1 gene can predispose individuals to hearing loss when administered with the aminoglycoside antibiotics, widely used in treatment of TB.
Correction of the gene defect underlies the genetic cause of hearing loss, and therefore gene therapy and genome editing have been touted as one of the possible emerging therapies for hereditary or genetic causes of deafness. Gene therapy typically involves replacing or supplementing a dysfunctional gene with normal or functional genes. There are a number of molecular approaches that have been widely used for such replacement or supplementation.
In addition to viral vectors, advanced non-viral methods are revolutionising gene therapy for genetic hearing loss. Lipid nanoparticles (LNPs) facilitate gene delivery by encapsulating nucleic acids and fusing with cell membranes, enabling efficient and targeted gene transfer. Electroporation employs controlled electrical pulses to transiently permeabilize the cell membrane, allowing direct uptake of genetic material. Cutting-edge genome editing technologies, such as CRISPR-Cas9, enable precise modification of specific DNA sequences through RNA-guided endonuclease activity, allowing targeted gene disruption or correction. These methodologies collectively enhance the potential for precise, efficient, and lasting correction of genetic defects underlying hearing loss. One of the widely used approaches involves viral vectors, which can package large pieces of genetic material required to be delivered inside the cell (referred to as cargo). Adeno-associated virus (AAV) is one of the most well-studied and widely used vectors for this purpose. AAV offers several advantages: it is a safe vector, as it does not cause human diseases, and it can infect both dividing and non-dividing cells, thus having a broad spectrum of cells it can target for genetic editing.
In a recent report published in Nature Medicine, Chinese researchers provide early promise towards using gene therapy for at least one genetic hearing loss. Researchers at the Fudan University, in collaboration with a number of research and clinical centres in China, proposed that gene therapy could effectively treat a form of genetic deafness involving the OTOF gene, known as hereditary deafness 9. Mutations in the OTOF gene account for approximately 2-8% of all genetic hearing loss cases. In this clinical trial, researchers employed Adeno-associated virus vectors with the intention of inserting a healthy OTOF gene into patients’ ears using a harmless virus. All patients experienced improved hearing in both ears. Initially performed on one ear, the study was expanded to test bilateral (both ears) therapy in five paediatric patients.
The researchers in the report suggest that no severe side effects were observed, while among the recorded 36-odd minor side effects, the most common were increased lymphocyte counts and cholesterol levels apart from an increase in lactate dehydrogenase levels, which is a marker for tissue damage in the body. Hearing tests showed significant improvement in all patients reported and all patients regained the ability to understand speech and locate sound sources. The promising results indicate that AAV gene therapy is safe and effective for treating hereditary deafness.
While the initial results are encouraging, Adeno-associated virus vectors come with their own set of caveats. The foremost being that our immune system can recognise and eliminate the virus making it less effective in individuals who are immunised, and also limits the re-administration of the gene therapy vector, since the primary administration would produce antibodies against the virus. Previous studies have suggested that approximately one-fifth to one-third of the patients have neutralising antibodies against AAV.
The present report is limited by the small number of patients studied and reported over a short follow-up period. However, it is encouraging that the clinical trial is ongoing and longer-term follow-up data of the patients would be available soon. While the results are encouraging and provide immense hope, we are not yet on a firm ground to assert that gene therapy for hearing loss is paving the way towards a sound future.
(Vinod Scaria is a consultant at Vishwanath Cancer Care Foundation, and Rahul Bhoyar is a senior scientist at Karkinos Healthcare. Opinions are personal)
month
Please support quality journalism.
Please support quality journalism.