Fitness
Study Reveals Structure of Alzheimer’s Molecules in Human Brain – Neuroscience News
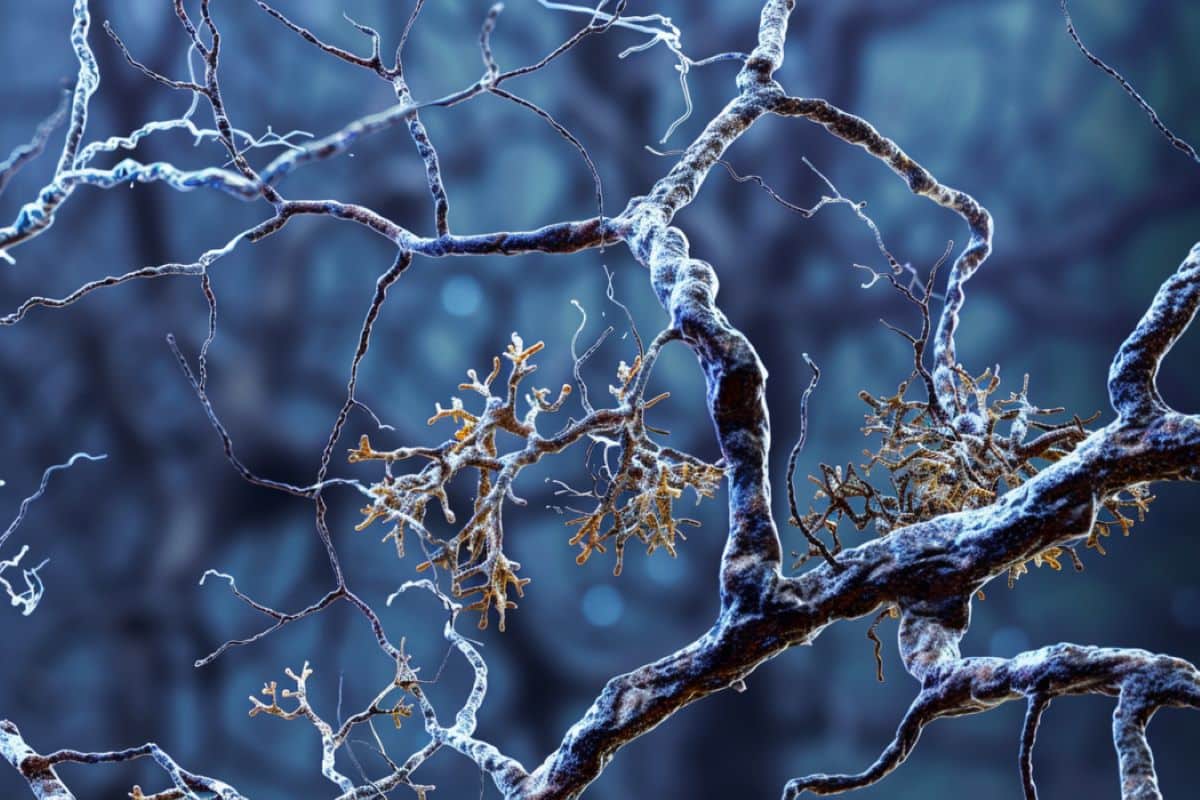
Summary: In a groundbreaking study, scientists have determined the structure of molecules within a human brain affected by Alzheimer’s disease. Using cryo-electron tomography and fluorescence microscopy, researchers created 3D maps of proteins like β-amyloid and tau, which cause dementia.
This study provides new insights into how these proteins disrupt cellular communication, leading to Alzheimer’s symptoms. The findings may pave the way for novel treatments for neurological diseases.
Key Facts:
- Innovative Imaging: Cryo-electron tomography and fluorescence microscopy used to map proteins.
- Key Proteins: Focus on β-amyloid plaques and tau filaments, crucial in Alzheimer’s.
- Future Treatments: Insights may lead to new therapeutic targets for Alzheimer’s and other neurological diseases.
Source: University of Leeds
Scientists investigating Alzheimer’s disease have determined the structure of molecules within a human brain for the very first time.
Published today in Nature, the study describes how scientists used cryo-electron tomography, guided by fluorescence microscopy, to explore deep inside an Alzheimer’s disease donor brain.
This gave 3-dimensional maps in which they could observe proteins, the molecular building blocks of life a million-times smaller than a grain of rice, within the brain.
The study zoomed in on two proteins that cause dementia– ‘β-amyloid’, a protein that forms microscopic sticky plaques, and ‘tau’ – another protein that in Alzheimer’s disease forms abnormal filaments that grow inside cells and spread throughout the brain.
This study revealed the molecular structure of tau in tissue, how amyloids are arranged, and new molecular structures entangled within this pathology in the brain.
Dementia is the leading cause of death in the UK, with Alzheimer’s its most common form.
In Alzheimer’s disease, both β-amyloid plaques and abnormal tau filaments are thought to disrupt cellular communication, which leads to symptoms such as memory loss and confusion, and cell death.
Dr Rene Frank, lead author and Associate Professor in the University of Leeds’s School of Biology, said: “This first glimpse of the structure of molecules inside the human brain offers further clues to what happens to proteins in Alzheimer’s disease but also sets out an experimental approach that can be applied to better understand a broad range of other devastating neurological diseases.”
Over the past 70 years a vast catalogue of molecular structures has been accumulated by several thousand scientists across the globe, each working with proteins in isolation in a test tube. However, it has long been known that most functions in biology are the consequence of an orchestra of many different proteins.
This study carried out at the University of Leeds in collaboration with scientists at Amsterdam UMC, Zeiss Microscopy, and the University of Cambridge, is part of new efforts by structural biologists to study proteins directly within cells and tissues, their native environment – and how proteins work together and affect one another, particularly in human cells and tissues ravaged by disease.
In the longer-term it is hoped that observing this interplay of proteins within tissues will accelerate identifying new targets for next generation mechanism-based therapeutics and diagnostics.
About this Alzheimer’s disease research news
Author: Leanne Dewsnap
Source: University of Leeds
Contact: Leanne Dewsnap – University of Leeds
Image: The image is credited to Neuroscience News
Original Research: Open access.
“CryoET of β-amyloid and tau within post-mortem Alzheimer’s disease brain” by Rene Frank et al. Nature
Abstract
CryoET of β-amyloid and tau within post-mortem Alzheimer’s disease brain
A defining pathological feature of most neurodegenerative diseases is the assembly of proteins into amyloid that form disease-specific structures.
In Alzheimer’s disease, this is characterized by the deposition of β-amyloid and tau with disease-specific conformations. The in situ structure of amyloid in the human brain is unknown.
Here, using cryo-fluorescence microscopy-targeted cryo-sectioning, cryo-focused ion beam-scanning electron microscopy lift-out and cryo-electron tomography, we determined in-tissue architectures of β-amyloid and tau pathology in a postmortem Alzheimer’s disease donor brain. β-amyloid plaques contained a mixture of fibrils, some of which were branched, and protofilaments, arranged in parallel arrays and lattice-like structures.
Extracellular vesicles and cuboidal particles defined the non-amyloid constituents of β-amyloid plaques. By contrast, tau inclusions formed parallel clusters of unbranched filaments.
Subtomogram averaging a cluster of 136 tau filaments in a single tomogram revealed the polypeptide backbone conformation and filament polarity orientation of paired helical filaments within tissue. Filaments within most clusters were similar to each other, but were different between clusters, showing amyloid heterogeneity that is spatially organized by subcellular location.
The in situ structural approaches outlined here for human donor tissues have applications to a broad range of neurodegenerative diseases.










